The current coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has resulted in approximately 5.42 million deaths and over 286 million confirmed cases. The first instances of COVID-19 were reported in Wuhan, Hubei Province, China, in December 2019, with the majority of those affected having worked in a seafood and animal wholesale market. The patients had a fever, cough, chest discomfort, and pneumonia, which necessitated hospitalization and the use of ventilators for assistance, with some of them dying.
Patients’ samples were submitted for isolation in cell culture, followed by RT-qPCR and next-generation sequencing (NGS), indicating coronavirus (CoV) as the causal agent. Immune dysregulation, gastrointestinal sickness, and long-term post-COVID-19 disorders have all become clinical symptoms since then. There is now no cure or preventative treatment to prevent infection, but various vaccines have been developed and distributed over the world to minimize rates of severe disease and mortality. SARS-CoV-2 variants have recently emerged, raising concerns about vaccine and treatment efficacy.
There’s also been a lot of interest in figuring out how infection affects pregnancy and fetal development, but there are still a lot of unknowns. As a result, a review conducted by a team of researchers from the Icahn School of Medicine at Mount Sinai, the University of Colorado, and Active Motif, Incorporated compiles information on SARS-CoV-2 virology, in utero transmission from infected pregnant mothers to fetuses, new findings on possible methods of SARS-CoV-2 cellular trafficking through exosomes, and the transcriptomic effects of SARS-CoV-2 infection, in order to inform future studies aimed at a better understanding of COVID-19 and the development of therapeutic solutions against SARS-CoV-2.
This review article is published in the Journal of Developmental Biology.
The study
SARS-CoV-2 was discovered to be a member of the Coronaviridae family, of the genus betacoronavirus (which includes MERS-CoV, which was the causative agent in the Middle East respiratory disease outbreaks in 2012), and the subgenus sarbecovirus, of which SARS-CoV (associated with the 2002–2003 pandemic and first identified in the Guangdong Province, China) is also a member. Even though SARS-CoV-2 and SARS-CoV are both sarbecoviruses, SARS-CoV-2 was discovered to be more closely related to other bat SARS-like betacoronaviruses. Bats have been postulated as the virus’s reservoir because of their genetic similarity; other animals, such as pangolins, have been proposed as potential intermediate hosts because of their genomic sequence similarity.
Reported impacts of SARS-CoV-2 infection on fetuses through pregnant mothers, and on children. Abbreviations: MISC multisystem inflammatory syndrome in children. “?” indicates that transmission mechanism is not clearly understood.
COVID-19 transmission from infected pregnant mothers to fetuses has been documented, albeit infrequently. COVID-19 instances in children have been recorded, with fatal results. Long COVID, a severe infection outcome in which symptoms last for five weeks or longer after an acute SARS-CoV-2 infection, has been described in children in the same way it has been in adults. Children have also been diagnosed with pediatric inflammatory multisystem syndrome (PIMS-TS), which has been linked to COVID-19. Compared to older children and adults, children under the age of 5 with mild to moderate COVID-19 have more SARS-CoV-2 viral RNA in their nasopharynx, which could affect transmission.
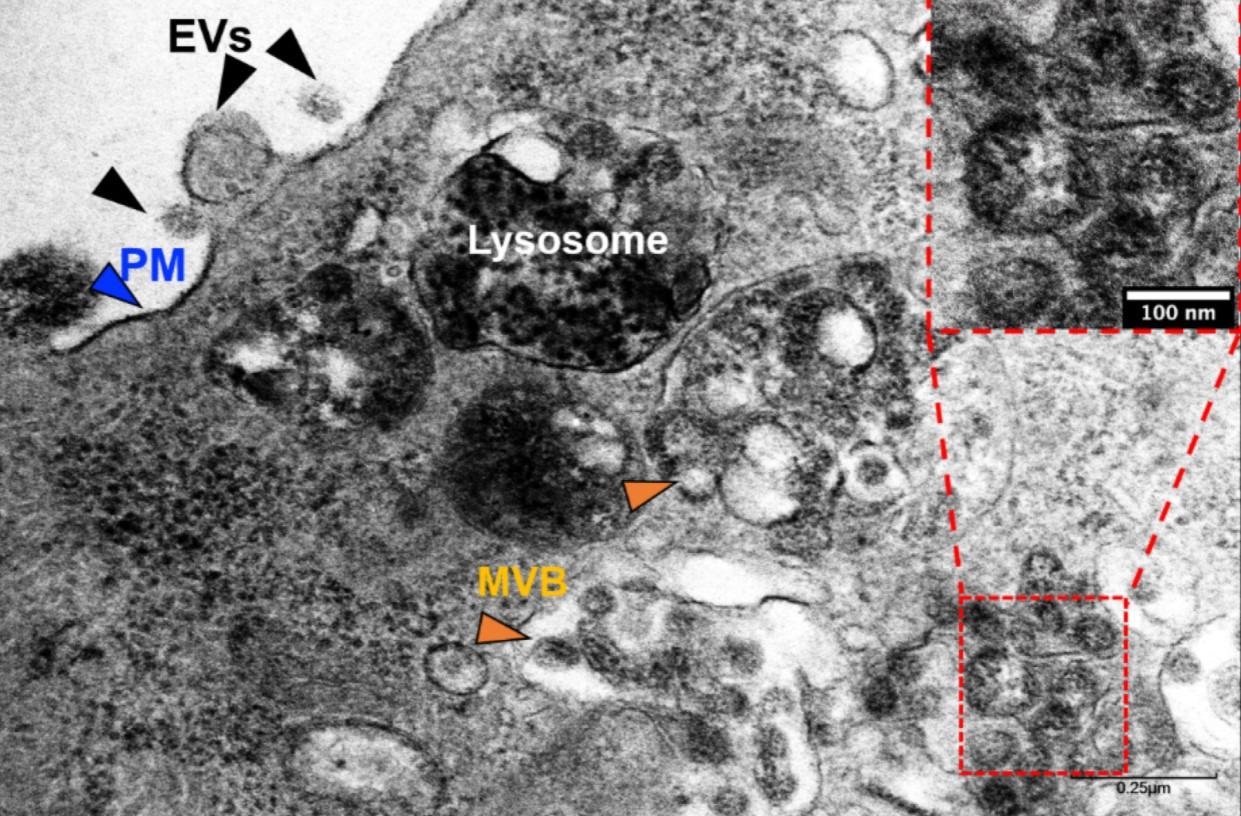
Transmission electron microscopy (TEM) of SARS-CoV-2-infected Vero E6 cells. Exosomes and/or a SARS-CoV-2-like particle can be seen (inset) inside an MVB. Evs—extracellular vesicles, PM—plasma membrane, MVB—multivesicular bodies.
Infection with SARS-CoV-2 causes fetal discomfort as well as significant morbidity and death in infants. There is insufficient evidence indicating negative impacts on future generations resulting from persons who tested positive for COVID-19 during pregnancy. However, the findings of multi-system inflammatory syndrome in children (MIS-C) and other complications suggest that more research is needed to fully understand the full range of COVID-19 effects in children, in utero development, and on SARS-CoV-2 cellular trafficking mediated by exosomes during the in utero and perinatal developmental stages.
Although it is apparent that SARS-CoV-2 infection triggers an immune response in pregnant women, the effects on fetal immune responses are still a hot topic of discussion. A recent study looked into 205 babies born to COVID-19-positive mothers. While only 10% of newborns tested positive for COVID-19, the majority of SARS-CoV-2-infected infants produced immunoglobulin G and M (IgG, IgM) antibodies. No viral RNA was found in the placentas of COVID-19 positive pregnant women in another study.
Furthermore, there appear to be no verified examples of SARS-CoV-2 infection transmitted from mothers to their fetuses during pregnancy. Although severe sickness has been documented in infants under the age of one year, such cases have had underlying comorbidities established. These data show that vertical infection is uncommon and that infants born to COVID-19-positive women have innate passive immunity.
Exosomes are released by every cell type that has been investigated so far. Exosomes from the mesenchymal, endothelial, and trophoblastic lineages have been studied in relation to the placental lineage and have been shown to decrease T-cell expression. The role of exosome trafficking in utero and its significance in SARS-CoV-2 infections and the subsequent establishment of an immune response in newborns were examined in this study.
Exosomes are endocytic-derived extracellular nanovesicles that package cellular contents. They are thought to help maintain cellular homeostasis, although the mechanism for their formation is unknown. SARS-CoV-2 infections via exosomes or in utero immunity development appear to be two possible ideas for exosomal contribution in utero and fetal development. While viral RNA has been identified in exosomes, there appears to be little to no viral replication in gestation. This finding refutes the first notion that exosomes could cause viral infection in the womb.
Implications
The effects of SARS-CoV-2 on the transcriptome provide a wealth of data that must be effectively utilized. The transcriptomic profiles reveal which genes are upregulated or downregulated by the infection, allowing them to be therapeutically targeted to reverse the effect. These profiles can also be used as biomarkers to determine the severity of infection, ranging from mild to severe. Finally, these profiles provide additional insight into how SARS-CoV-2 infection affects cellular development and programming.
To fill knowledge gaps in understanding maternal-fetal transmission mechanisms, inheritance patterns of epigenetic imprints can be compared between cells from COVID-19-infected mothers and their offspring. It will be interesting to see if the mother’s epigenetic changes caused by SARS-CoV-2 infection are passed down to her offspring. Overall, this review aims to broaden the perspective on several elements of COVID-19, which will aid in the understanding of other viral infections and help us better prepare for future outbreaks.













